Amines & its Classifications - Compounds Containing Nitrogen - Chemistry Class 12 | Summary and Q&A
TL;DR
This video discusses the classification of amines, including primary, secondary, tertiary, and quaternary, based on the replacement of hydrogen atoms in ammonia with alkyl or aryl groups.
Key Insights
- ❓ Amines are organic derivatives of ammonia, and amides are a subset of amines.
- 🫀 Amines can be classified as primary, secondary, tertiary, or quaternary based on the number of hydrogen atoms replaced.
- 🫀 Secondary amines can be further classified as simple or mixed, depending on the similarity of the alkyl groups attached to the nitrogen atom.
Transcript
click the bell icon to get latest videos from equator references the previous lectures we have discussed about the Nitro alkane and that is nothing but the compounds that contain nitrogen and now we are going to talk about the mixture that is our minds so now let us understand what our minds every what is the classification of our minds can this to... Read More
Questions & Answers
Q: What are amides and how are they related to amines?
Amides are organic derivatives of ammonia in which one or more hydrogen atoms attached to nitrogen are replaced by alkyl or aryl groups. They are related to amines as amines are a subset of amides, specifically the compounds in which all the hydrogen atoms of ammonia are replaced by alkyl or aryl groups.
Q: How are amines classified?
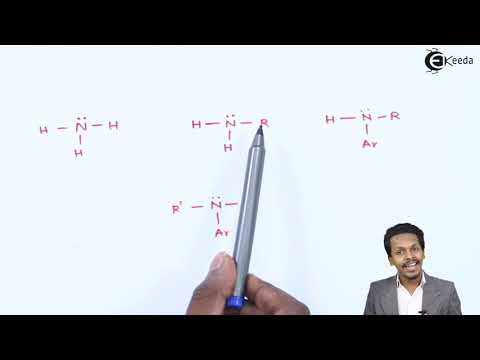
Amines are classified as primary, secondary, tertiary, or quaternary, based on the number of hydrogen atoms replaced by alkyl or aryl groups in ammonia. Primary amines have one hydrogen atom replaced, secondary amines have two, tertiary amines have three, and quaternary amines have all four hydrogen atoms replaced.
Q: What is the difference between simple secondary amines and mixed secondary amines?
Simple secondary amines are secondary amines in which both alkyl groups attached to the nitrogen atom are the same. Mixed secondary amines, on the other hand, have different alkyl groups attached to the nitrogen atom.
Q: How can the names of amines be determined?
The names of amines are determined based on the alkyl or aryl groups attached to the nitrogen atom. In naming, the names of the alkyl or aryl groups are combined with the word "amine." The names can be further specified to indicate whether the amine is primary, secondary, or tertiary.
Summary & Key Takeaways
-
Amides are organic derivatives of ammonia in which one or more hydrogen atoms attached to nitrogen are replaced by alkyl or aryl groups.
-
Amines can be classified as primary, secondary, tertiary, or quaternary, depending on the number of hydrogen atoms replaced by alkyl or aryl groups.
-
Secondary amines can further be classified as simple secondary amines or mixed secondary amines, depending on the similarity or difference of alkyl groups attached to the nitrogen atom.
Share This Summary 📚
Explore More Summaries from Ekeeda 📚